What parts of the protein are chemically bonded together to form the secondary structure?
Proteins structures are made past condensation of amino acids forming peptide bonds. The sequence of amino acids in a poly peptide is chosen its master construction. The secondary structure is determined past the dihedral angles of the peptide bonds, the 3rd structure by the folding of proteins chains in space. Association of folded polypeptide molecules to complex functional proteins results in quaternary structure.
Table of Contents
- Nomenclature of Proteins
- Primary Construction of Protein
- Secondary Construction of Protein
- 3rd Structure of Protein
- Quaternary Structure of Poly peptide
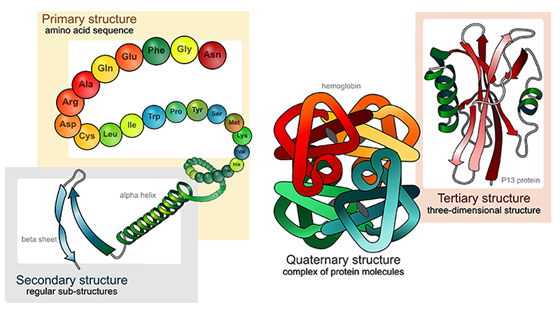
Main, Secondary, Third and Quaternary Structure of Proteins
Define Protein Construction
Poly peptide structure is divers equally a polymer of amino acids joined past peptide bonds.
Related Topics
- Amino acids
- Peptide bond
- Denaturation of Proteins
- Enzymes
- Hormones
Allow us see how a peptide bond is established from the following reaction:
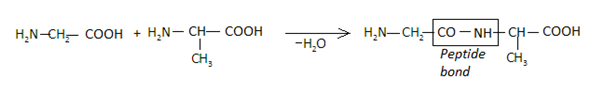
Formation of Peptide Bond
Nosotros can thus see that the peptide bond (-CO-NH) is formed betwixt the amine grouping of i molecule and the carboxyl group of the adjacent molecule followed by the emptying of a water molecule. This bond is otherwise an amide linkage. When peptide bonds are established amidst more than than ten amino acids, they together form a polypeptide chain. Very often, when a polypeptide chain has a mass exceeding 10000u and the number of amino acids in the chain exceeding 100, we become a poly peptide.
As well Read: Denaturation of Proteins
Recommended Videos

Classification of Proteins
Based on the molecular shape, proteins tin be classified into 2 types.
1. Gristly Proteins:
When the polypeptide chains run parallel and are held together past hydrogen and disulfide bonds, then the fiber-like structure is formed. Such proteins are mostly insoluble in water. These are water-insoluble proteins.
Instance – keratin (present in hair, wool, and silk) and myosin (nowadays in muscles), etc.
2. Globular Proteins:
This structure results when the chains of polypeptides scroll around to give a spherical shape. These are usually soluble in water.
Example – Insulin and albumins are common examples of globular proteins.
Levels of Poly peptide Structure
1. Primary Construction of Protein
- The Primary structure of proteins is the verbal ordering of amino acids forming their chains.
- The verbal sequence of the proteins is very important as information technology determines the last fold and therefore the role of the protein.
- The number of polypeptide bondage together form proteins. These chains have amino acids bundled in a detail sequence which is characteristic of the specific poly peptide. Whatever change in the sequence changes the entire protein.
The following picture represents the primary poly peptide structure (an amino acid chain). Equally yous might wait, the amino acid sequence within the polypeptide chain is crucial for the protein's proper functioning. This sequence is encrypted in the DNA genetic code. If mutation is present in the Deoxyribonucleic acid and the amino acid sequence is changed, the protein function may be affected.

Chief Construction of Protein
The protein 'due south primary construction is the amino acid sequence in its polypeptide chain. If proteins were popcorn stringers designed to decorate a Christmas tree, a protein 's chief construction is the sequence in which various shapes and varieties of popped maize are strung together.
Covalent, peptide bonds which connect the amino acids together maintain the primary construction of a protein. The following figure shows the primary insulin structure, which is the first poly peptide to be sequenced.
Notice the position of each amino acid numerated on the correct side of the figure. By convention, biochemists ofttimes list the amino acids that begin at the polypeptide chain'due south amino-terminus.
All documented genetic disorders, such as cystic fibrosis, sickle cell anemia, albinism, etc., are acquired by mutations resulting in alterations in the primary protein structures, which in plow lead to alterations in the secondary , tertiary and probably quarterly structure.
Amino acids are modest organic molecules consisting of a chiral carbon with four substituents. Of those simply the fourth the side concatenation is different among amino acids.
two. Secondary Structure of Protein
Secondary structure of protein refers to local folded structures that form within a polypeptide due to interactions between atoms of the backbone.
- The proteins do not exist in just simple chains of polypeptides.
- These polypeptide chains unremarkably fold due to the interaction between the amine and carboxyl group of the peptide link.
- The construction refers to the shape in which a long polypeptide chain can be.
- They are found to be in 2 different types of structures α – helix and β – pleated sheet structures.
- This structure arises due to the regular folding of the backbone of the polypeptide chain due to hydrogen bonding between -CO grouping and -NH groups of the peptide bond.
- However, segments of the protein chain may learn their own local fold, which is much simpler and usually takes the shape of a spiral an extended shape or a loop. These local folds are termed secondary elements and course the proteins secondary structure.

Secondary Structure of Protein
(a) α – Helix:
α – Helix is one of the most mutual means in which a polypeptide chain forms all possible hydrogen bonds by twisting into a right-handed spiral with the -NH group of each amino acid balance hydrogen-bonded to the -CO of the next turn of the helix. The polypeptide chains twisted into a right-handed screw.
(b) β – pleated sheet:
In this organisation, the polypeptide chains are stretched out beside 1 some other and so bonded by intermolecular H-bonds. In this construction, all peptide chains are stretched out to almost maximum extension and and then laid side by side which is held together by intermolecular hydrogen bonds. The structure resembles the pleated folds of curtain and therefore is known equally β – pleated sail
3. Tertiary Structure of Protein
- This structure arises from further folding of the secondary structure of the poly peptide.
- H-bonds, electrostatic forces, disulphide linkages, and Vander Waals forces stabilize this construction.
- The tertiary structure of proteins represents overall folding of the polypeptide bondage, further folding of the secondary construction.
- Information technology gives rise to two major molecular shapes chosen gristly and globular.
- The principal forces which stabilize the secondary and tertiary structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic forces of attraction.

Third Structure of Protein
4. Quaternary Structure of Poly peptide
The spatial arrangement of various 3rd structures gives rise to the quaternary structure. Some of the proteins are composed of two or more than polypeptide chains referred to every bit sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.

Quaternary Structure of Protein
The exact amino acrid sequence of each protein drives it to fold into its own unique and biologically agile 3-dimensional fold also known every bit the tertiary structure. Proteins consist of different combinations of secondary elements some of which are simple whereas others are more than complex. Parts of the protein chain, which have their own three-dimensional fold and can be attributed to some office are chosen "domains" . These are considered today as the evolutionary and functional building blocks of proteins.
Many proteins most of which are enzymes contain organic or elemental components needed for their activity and stability. Thus the study of protein development not simply gives structural insight but also connects proteins of quite different parts of the metabolism.
Besides Read: Laboratory Test of Proteins
Rules of Protein Construction
- The type determines the function of a protein.
- A protein's shape is determined past its primary structure (the amino acid sequence).
- The amino acid sequence inside a poly peptide is determined by the encoding sequence of nucleotides in the factor (Deoxyribonucleic acid).
Summary of Protein Construction
Linderstrom-Lang (1952) in detail first suggested a hierarchy of protein structure with four levels: primal, secondary, tertiary , and quaternary. Yous are already familiar with this hierarchy, because the near useful starting point for pedagogy bones protein structure is this structural grouping.
- The main construction of poly peptide is the bureaucracy'southward basic level, and is the detail linear sequence of amino acids comprising 1 polypeptide concatenation.
- Secondary construction is the side by side level up from the primary structure, and is the regular folding of regions into specific structural patterns within one polypeptide concatenation. Hydrogen bonds betwixt the carbonyl oxygen and the peptide bond amide hydrogen are normally held together by secondary structures.
- Tertiary construction is the next level up from the secondary structure, and is the item three-dimensional organisation of all the amino acids in a single polypeptide chain. This structure is normally conformational, native, and active, and is held together by multiple noncovalent interactions.
- 4th construction is the next 'step up' between ii or more polypeptide chains from the 3rd structure and is the specific spatial arrangement and interactions.
Ofttimes Asked Questions – FAQs
What makes upward poly peptide structure?
A poly peptide's main structure refers to the amino acid sequence in the polypeptide chain. Peptide bonds that are made during the poly peptide biosynthesis procedure hold the master construction together.
What are the four stages of protein structure?
Four levels of construction of proteins. The chief, secondary, tertiary and quaternary levels of protein construction are the four stages. To fully sympathize how a protein functions, information technology is helpful to sympathise the purpose and role of each level of protein structure.
What is the process of poly peptide folding?
The folding of proteins is the machinery through which a poly peptide construction assumes its functional shape or conformation. Both molecules of protein are heterogeneous unbranched amino acid chains. They may perform their biological function past coiling and folding in a detail three-dimensional shape.
How proteins are formed?
Amino acids form a polypeptide, another word for poly peptide when bound by a sequence of peptide bonds. The polypeptide and so folds into a particular conformation based on the interactions (strained lines) between its side chains of amino acids.
Is DNA a protein?
DNA is oftentimes associated with proteins in the nucleus called histones, but Dna itself is not a protein. No. Dna is a nucleic acid consisting of phosphate and sugar groups based on purine and pyrimidine, while proteins are large molecules made up of one or more long amino acid bondage.
What stabilizes protein structure?
Hydrogen bonding in the polypeptide chain and between amino acid "R" groups helps to preserve protein structure past keeping the poly peptide in the form formed by the hydrophobic interactions. What is chosen a disulfide bridge is formed by this sort of bonding.
What determines protein structure?
In the polypeptide chain, the principal structure of a poly peptide relates to the amino acid sequence. The main structure is jump together by peptide bonds that are made during the stage of protein biosynthesis. The primary construction of a protein is determined past the cistron corresponding to the poly peptide.
What is the primary structure of a protein?
The linear sequence of amino acids within a poly peptide is called the primary structure of the poly peptide. A sequence of just xx amino acids, each of which has a special side concatenation, is made upwards of proteins. The side chains of amino acids are chemically distinct.
We have thus gone through the structure of a protein in brief. For complete understanding, join BYJU'Southward.
Source: https://byjus.com/chemistry/protein-structure-and-levels-of-protein/
0 Response to "What parts of the protein are chemically bonded together to form the secondary structure?"
Post a Comment